For Doctors and Medical Professionals|What is private import of medicines?
This page is about the personal import of pharmaceuticals, specifically for Japanese doctors and medical professionals.
What is personal importation of pharmaceuticals?
Personal importation of pharmaceuticals refers to "purchasing pharmaceuticals from overseas distributors and importing them into Japan".
Normally, importation of pharmaceuticals falls under the category of "manufacture and sale" under the Pharmaceutical Affairs Law.
To import pharmaceuticals for business purposes, you will need either a marketing license, manufacturing license, or registration from the Minister of Health, Labor and Welfare.
However, the Ministry of Health, Labor and Welfare allows doctors to personally import pharmaceuticals regardless of whether or not they have been approved, in the following cases.
- For patient treatment purposes
- For research purposes
In this case, various procedures are required, including, in principle, the need to submit an application for import confirmation and other necessary documents to the regional health and welfare bureau and have an import confirmation certificate issued.
Medical Life Co., Ltd. provides personal import agent services exclusively for physicians and medical professionals.
We handle all import-related procedures on your behalf.
Please see below for details.
Flowchart for Personal Importation of Pharmaceuticals by Doctors
The following is a brief flow chart for physicians importing pharmaceuticals.
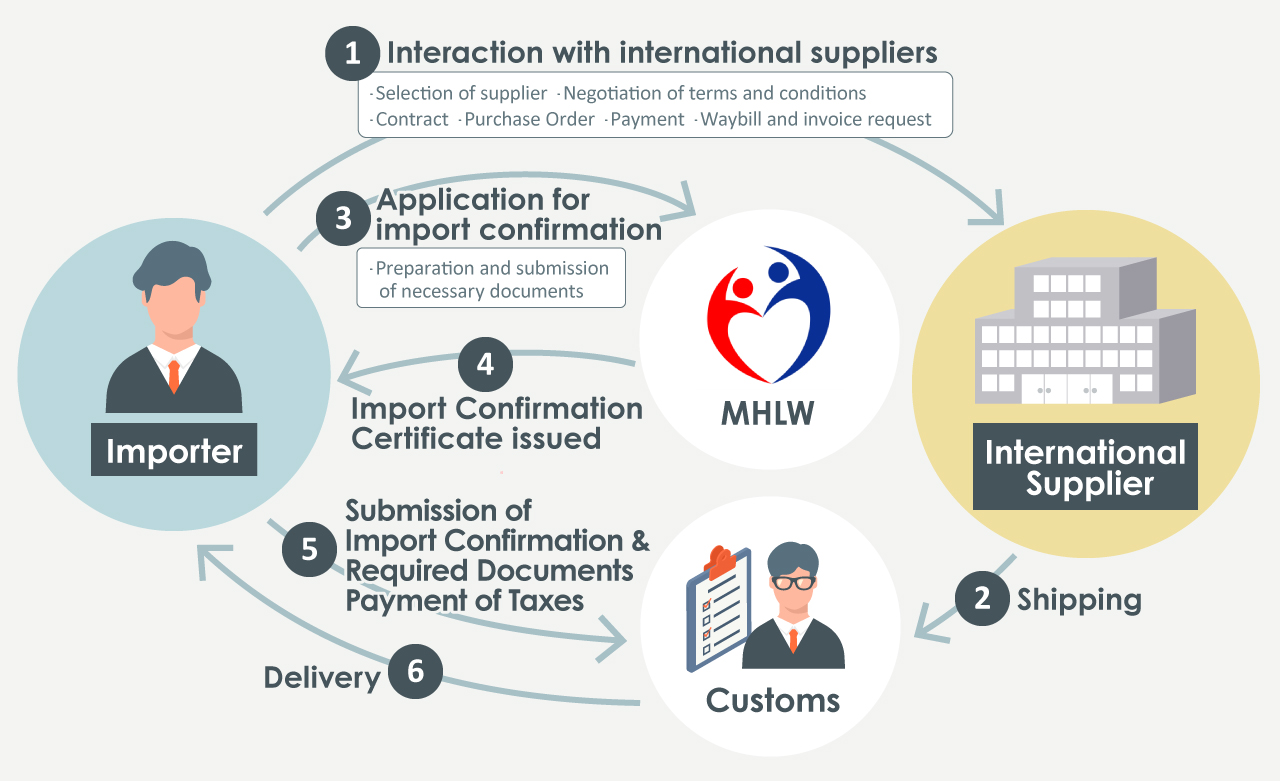
The basic flow of personal importation of pharmaceuticals consists of steps (1) to (6), which we will explain in more detail below.
Communication with overseas suppliers
The Interaction with overseas suppliers is wide-ranging.
Selection, negotiation, and contract
- Finding suppliers and conducting credit checks (checking government-issued licenses, financial information, etc.)
- Establishment of various terms and conditions (such as transaction terms, payment terms) and warranty coverage.
- Establishment of trading accounts
etc.
- Negotiating with multiple suppliers on price.
Negotiating with multiple suppliers on price.
- Sending a purchase order with all specified terms and conditions
- Progress management of suppliers
- After notification of shipping, request issuance of waybill/invoice (necessary for import preparation)
Payment
Payment is due by the due date after receipt of invoice.
- Payment due dates vary by supplier.
Import Preparation
- Submit the required documents to the Ministry of Health, Labor, and Welfare to receive the Application for Import Confirmation (Yakkan Shoumei)
- Submit the Application for Import Confirmation (Yakkan Shoumei) and documents to customs
- Payment of taxes
After the above, the product is finally delivered.
However, as the shipment originates from overseas, there's a potential for damage or other issues.
Furthermore, there is no guarantee that 100% of the goods will clear customs once the documents are submitted.
Precautions when Importing Medicines
The main precautions when importing pharmaceutical products are as follows.
- Medicines prohibited from import
- Potential issues or complications
- Safety of Medicines
Medicines prohibited from import
Narcotics, psychotropic drugs, stimulant materials, stimulants, marijuana, and designated drugs.
Other drugs and pharmaceutical raw materials that cannot be imported based on the Washington Convention, and those that fall under the category of intellectual property right violation goods cannot be imported.
Potential issues or complications
Trouble with overseas manufacturers, delivery trouble, trouble related to customs clearance, etc.
There are various potential issues.
Safety of Pharmaceuticals
There is a possibility that the imported medicine was manufactured in an unsanitary place or is counterfeit.
To ensure safety, Medical Life Co., Ltd. investigates suppliers and independently evaluates ingredients.
We handle imports on your behalf by anticipating various problems and taking thorough measures to ensure that your products are delivered safely and securely.
Isn't it illegal to import medicines privately?
The Ministry of Health, Labor, and Welfare allows physicians to import medicines from abroad for patient treatment or research purposes.
In other words, it is not illegal.
However, using imported pharmaceuticals for purposes other than their intended import purpose, such as selling or transferring them to a third party, is illegal.
Physicians can partner with pharmaceutical import agents like us without any issues.
Let's take a look at the scope of import agency services.
Scope of Import Agent Services
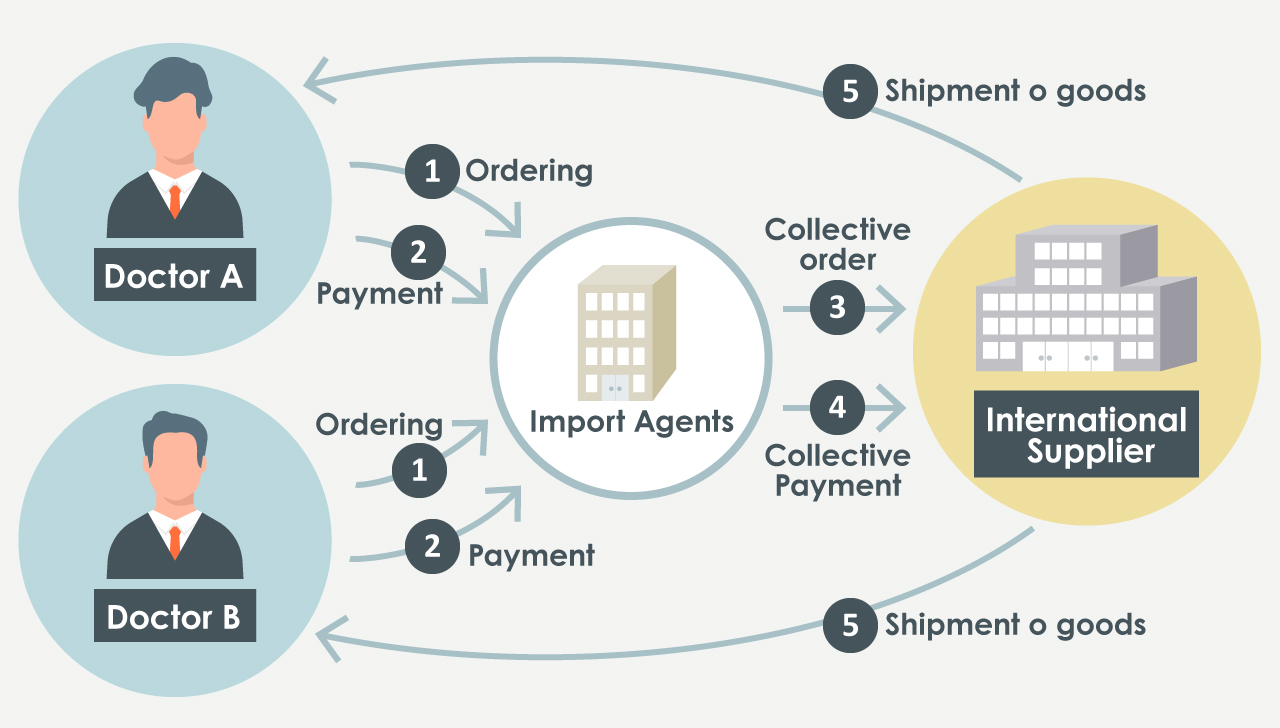
Upon receiving a request from the importer (physician), an import agent performs all import procedures on behalf of the importer, such as placing orders with suppliers and making payments.
Since the import agent solely facilitates the import procedures on behalf of the importer, the medicines sourced from overseas should be directly delivered to the importer, who is the physician.
It is considered illegal if the imported medicines are routed through the import agent before reaching the physician.
Furthermore, according to the Pharmaceuticals and Medical Devices Act, import agents are prohibited from advertising or promoting the product name, ingredient name, efficacy, price, usage method, etc. of pharmaceuticals that have not been approved in Japan.
Below, we'll examine both the permitted activities and those considered as violations in the business operations of import agents.
Cases that are not illegal
Cases that are illegal
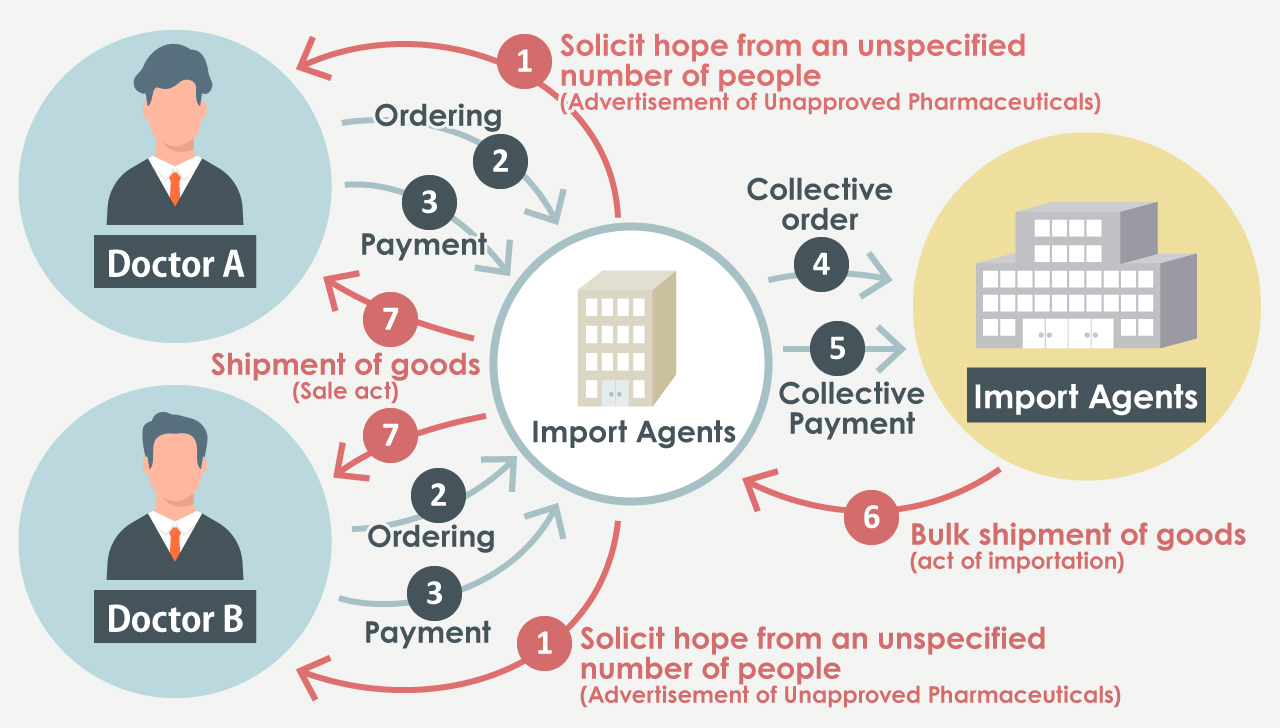
- The sections highlighted in red indicate the violations.
- Violation of Article 55, Paragraph 2 of the Pharmaceuticals and Medical Devices Act (prohibition of sale and award of counterfeit pharmaceuticals) and Article 68 of the Pharmaceuticals and Medical Devices Act (prohibition of advertisement of pharmaceuticals, medical devices and regenerative medicine products prior to approval).
Before engaging an import agent, it's crucial to understand the scope of their services in advance.
This precaution is necessary as you might face issues if you work with an import agent involved in illegal activities.
Medical Life Co., Ltd. complies with all laws and regulations and imports pharmaceuticals on behalf of our clients.
We promise to be a safe and secure import agent.
What if want to know more about importing pharmaceuticals?
Please don't hesitate to reach out with any concerns or questions, no matter how trivial.
If you have any concerns about importing pharmaceuticals, please feel free to contact Medical Life Co., Ltd.
Reference Articles
This page was created with reference to the following.
For those who intend to purchase pharmaceutical products from overseas|Ministry of Health, Labour and Welfare
Beware of health hazards and other risks! Personal import of medicines from overseas|Government Public Relations Online
Personal import of medicines and cosmetics (Customs Answer)
Guidance and Control of Personal Import Agents|Ministry of Health, Labour and Welfare
Act on Quality, Efficacy and Safety Assurance of Pharmaceuticals and Medical Devices, etc. | e-Gov Laws and Regulations Search








